How Is the Electronegativity Trend Related to the First Ionization
How IS The electronegativity trend relates to the first ionization energy trend. And this question we have to talk about the trend in the periodic table and period And periodic table particularly for period three.

Periodic Trends Webquest And Graphing Ps1 1 Graphing Ionization Energy Electron Affinity
The pressure of a gas is independent of the temperatured.
. How is the electronegativity trend related to the first ionization energy trend. Electronegativity values generally increase from left to right across the periodic table. Since having more tendency to attract an electron means that the atom has less tendency to give an electron more electronegative atoms require more energy to ionize.
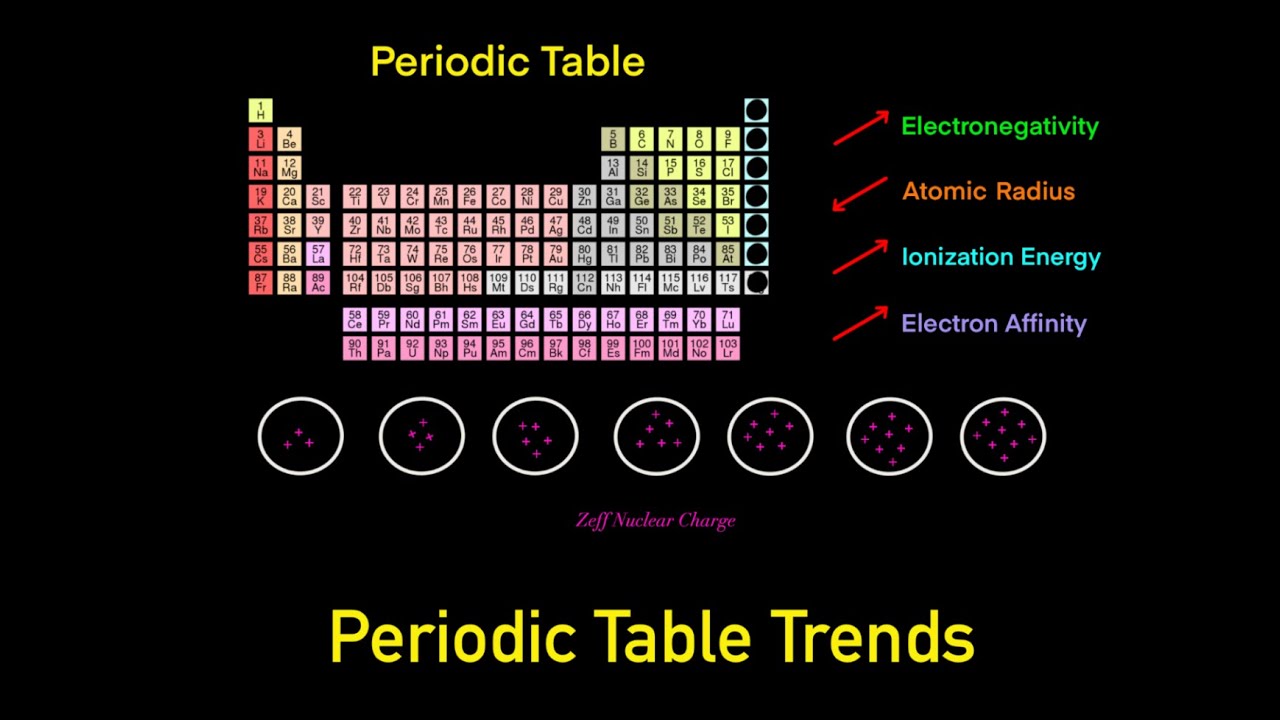
As we move across a period from left to right the nuclear charge increases and the atomic size decreases therefore the value of electronegativity increases across a period in the modern periodic table. Here is a look at the periodic table trends of electronegativity atomic radius electron affinity metallic character and ionization energy. There is extra stability when a type of orbital is half filled or completely filled.
The pressure of a gas is dependent on the volumeg. This is because the electrons are being held in closer to the protons which have opposing charges and therefore hold on to them in an atom with a small radius. Electronegativity decreases as you move up the table whereas ionization energy increases.
Here is a chart of electronegativity from Wikipedia. Electronegativities generally decrease from top to bottom of a group. Electronegativity increases as you move across the periodic table from left to right.
The greater pull increases the amount of electronegativity due to the pull of the electrons. Which of these statements are true. According to the elements of main group the first ionization energies generally decreases from top to bottom across the periodic table.
Electronegativity and first ionization energy both increase going up the Periodic Table. Electronegativity increases as you move up the table whereas ionization energy decreases. In contrast the ionization energy is the energy needed to remove an electron from an atom.
Electronegativity is a measure of the ability of an atom to attract the electrons when the atom is part of a compound. Why is the second ionization energy greater than the first ionization energy. My understanding is that elements with a higher electronegativity will be more reactive than elements with a lower electronegativity and that elements with a low ionization energy will be more reactive than elements with a high ionization energy.
This occurs due to a greater charge on the nucleus causing the electron bonding pairs to be very attracted to atoms placed further right on the periodic table. Gases fill their containers completelyc. The smaller the radius the higher the ionization energy.
The highest electronegativity value is for fluorine. January 10 2012 Posted by Dunee. Fluorine is the most electronegative element.
Electronegativity is the ability of an atom to pull electrons towards it. Actually the trend of electronegativity and first ionization energy is the same but just keep In mind there are several exceptions for example the first ionization energy of N is actually larger than O. Electronegativity is practically the opposite of ionization energy - it increases as the atomic radius gets smaller since then its protons are closer to its electrons and thus are able to exert more attractive force on each other.
This makes the recurring element properties noticeable in this table. Log in Sign up. Atomic radius Ionization energy electron affinity ionic radius electron negativity valence electrons 10 Terms leighhardy1999 Ionization EnergyAtomic RadiusElectronegativity 9 Terms.
The amount of energy required to separate one electron from its atom first ionization energy depends on how tightly held the electron is. The electronegativity χ describes the ability of an atom to attract electrons towards itself. Electronegativity and first ionization energy both decrease as you move up the periodic table.
It shows how an atom can swiftly form a chemical bond. Across the periodic table. Will mark brainliest26.
This shows electronegativity decreasing as reactivity increases down. Gas pressure results from the collisions between gas particlesh. Ionization always requires energy.
Periodic Trends Learn with flashcards games and more for free. The key difference between electronegativity and ionization energy is that electronegativity explains the attraction of electrons while ionization energy refers to the removal of electrons from an atom. Periodic Trends in the Electronegativities of Elements.
If the radius is larger then those electrons on the outer edge of the atom arent being held in so close and are easier to lose - requiring a lower amount. Okay so in period three when we go from left to right and we go from left to right we can see that first of. Atoms are the building blocks of all existing substances.
The trend in ionization energy refers to how ionization energy follows a notable trend across the periodic table of the elements. For example the electronegativity trend across period 3 in the periodic table is depicted below. This depends on the number of protons and on the orbitals that the electron occupies.
Ionization energy typically increases as you move left or right across a row or element period and it typically decreases as you move top to bottom down a column or element group. Electronegativity is an atoms tendency to attract an electron.

What Did You Do Today At School Electronegativity Trends 3d Periodic Table Chemistry Activities Teaching Chemistry High School Science Activities
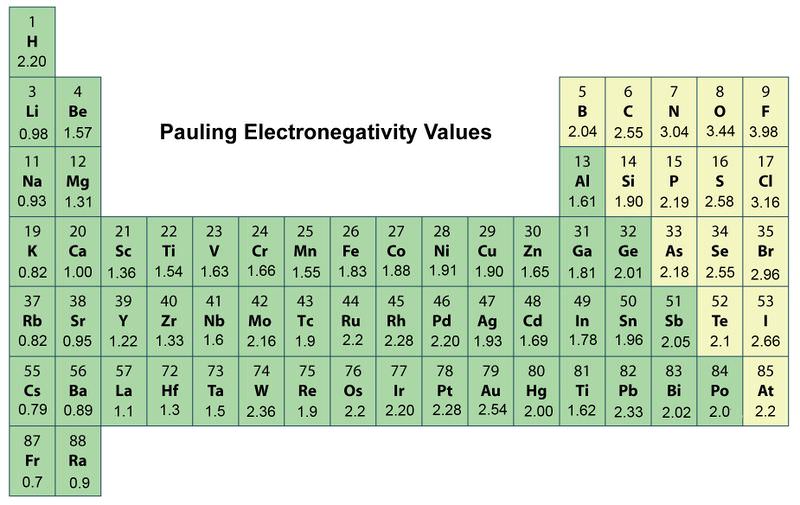
Periodic Trends In Electronegativity Ck 12 Foundation
Ionization Energy And Electronegativity

File Periodic Trends Svg Wikipedia The Free Encyclopedia Chemistry Classroom Chemistry Chemistry Lessons

Trends In The Periodic Ta Le Ionization Energy Atomic Radius Electron Affinity Electronegativity Ionization Energy Electron Affinity Electrons

Periodic Table Trends Chemistry Classroom Ionization Energy Middle School Literacy

Periodic Table Trends Trick Electronegativity Atomic Radius Ionization Energy Electron Affinity Youtube
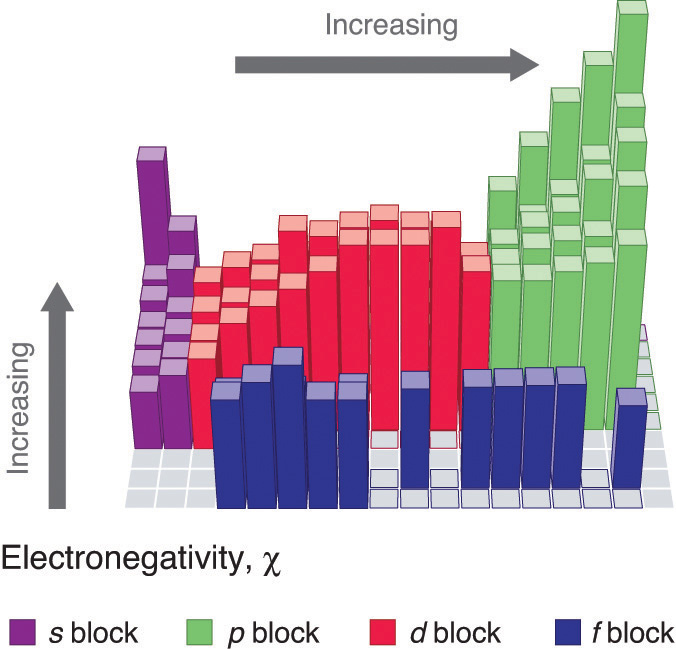
2 11 Trends Of The Periodic Table Chemistry Libretexts

8 4 Ionization Energy Ionization Energy Chemistry Chemistry Experiments

Periodic Property Deviations In The Trend Of Ionization Energy Ionization Energy Science Anchor Charts Study Materials

How Is Electronegativity Related To Ionization Energy And Electron Affinity Quora

Periodic Trends Made Easy Chemtalk

3 D Electronegativity Table Chemistry Activities Physical Science Chemistry Lessons

Easy To Use Chart Of Periodic Table Trends Teaching Chemistry Chemistry Classroom Science Chemistry

Periodic Trends Chemwiki Ionization Energy Chemistry Electron Affinity

Periodic Trends Chemwiki Electron Affinity Ionization Energy Chemistry Lessons
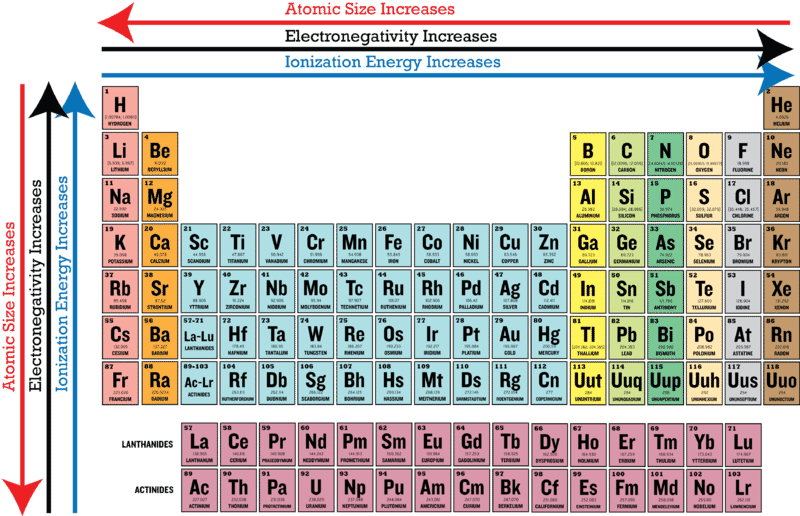
Periodic Trends In Electronegativity Ck 12 Foundation

Pin By Katie Rose On My Saves Periodic Table Ionization Energy Chemistry Lessons

Comments
Post a Comment